Stem cells systemically – an innovative therapeutic approach to the butterfly disease (EB)
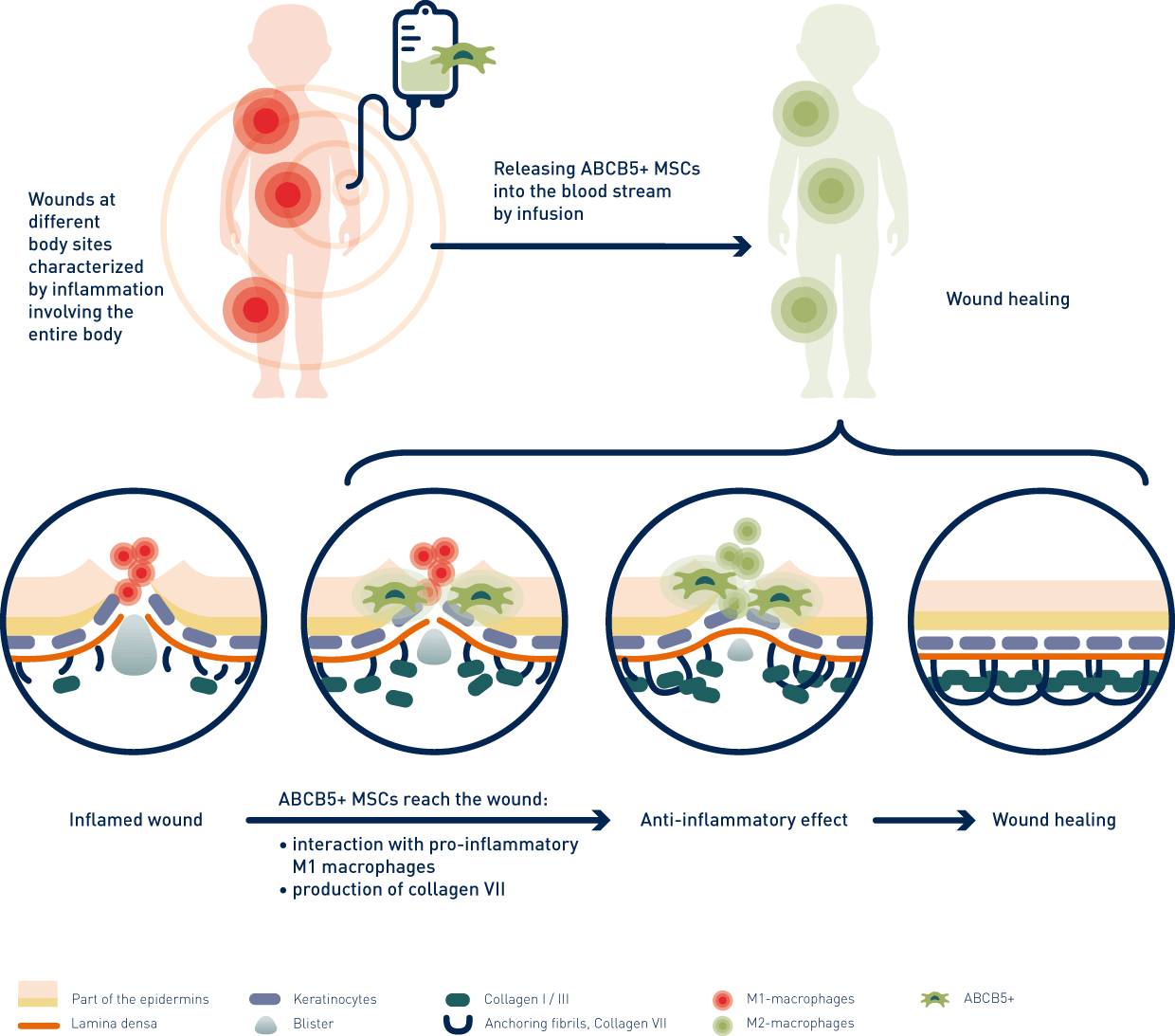
Due to their immunomodulatory and anti-inflammatory properties, ABCB5+ mesenchymal stem cells (ABCB5+ MSCs) may represent a new, promising therapeutic approach to EB. The cell therapy agent has already shown in chronic venous wounds (CVU) that the cells interact locally with the immune system and can thus enable the body to close chronic wounds1. Based on these data, the Paul-Ehrlich-Institute granted national market approval for external use in CVU patients in autumn 20212.
Since EB is a systemic, multi-organ disease, blistering and open wounds can occur anywhere in the body, including the inner mucous membranes (mouth, esophagus, or intestines).
Therefore, the stem cell therapy is not applied externally on the wounds but administered as an infusion. Because of the systemic effect via blood, stem cells can migrate to the injured tissue sites – externally as well as internally – nest in the wound (homing) and promote their healing.